Designs on field testing of gene drive for malaria control

As our programme of research and development steadily progresses in the lab, we have also been thinking about how best to test our gene drive technology in field trials. Over the last two years, we were fortunate to harness the support of FNIH to help us address this question. They convened a collaborative group that included the leading three developers of self-sustaining, or “low-threshold,” gene drive for malaria vector control: Target Malaria, Transmission Zero, and UCMI. The outputs from that group have now been crystalised into a paper that has just been published in Malaria Journal entitled: Considerations for first field trials of low-threshold gene drive for malaria vector control.
Despite the positive impacts of Long-Lasting Insecticidal Nets (LLINS) and IndoorResidual Spraying (IRS), sustainable reductions in malaria transmission require the development of innovative tools, such as genetic biocontrol of the Anopheles mosquito. Target Malaria has been pursuing the population suppression approach for gene drive, where the gene drive system introduces female sterility into Anopheles target populations leading to a reduction in the number of female mosquitoes in the population. Unlike LLINS or IRS, for example, self-sustaining gene drive is expected to spread and persist indefinitely in target populations. This property means our approach offers unique opportunity and strengths in malaria vector control.
We expect that gene drive:
- will require minimal numbers of mosquitoes in releases to achieve efficacy in the field
- could be effective in controlling malaria without the need for changes in human behaviour, unlike use of bednets, for example
- could be deployed to help reduce transmission rates in high transmission areas, particularly in areas where other more human-intensive delivery methods are challenging
- could be deployed in low transmission areas to help accelerate malaria elimination
- could be used to help prevent reintroduction and transmission in areas that have been declared malaria-free.
The first and most obvious question that one might ask when thinking about a field trial is: what do we need to test?! A key aspect of the work described in our new paper was the recognition that the use of gene drive for malaria control involves what we have called a “causal pathway.” And this causal pathway is what needs to be tested:
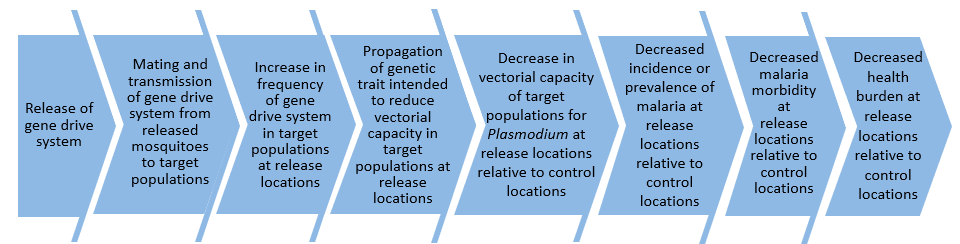
There is a wealth of previous experience in testing new interventions like LLINs for malaria control in the field. Typically, new interventions are first tested in small-scale field trials with simple objectives, for example, to establish whether they reduce mosquito biting rates in a small number of experimental huts. If these are successful, the next step is typically to evaluate the direct impact on malaria transmission in much more expensive field trials, called cluster randomised control trials (cRCTs), that can involve large numbers of human volunteers.
While the expected spread and persistence of gene drive is one of its key strengths, it also means there is more to consider for the design of the first field trials than for more conventional interventions. For example, ideally, the design of the first field trials might involve only one or two release locations and have only simple goals, like measuring the rate of increase in the proportion of mosquitoes carrying the gene drive transgene. However, any subsequent and larger trials of gene drive that might evaluate the impact on malaria could be affected by “spillover” effects from the earlier releases of our gene drive mosquitoes.
Therefore, even before the first field trials can be designed, we need to know already what those larger trials might look like, what their purpose is, and how they will measure impacts on malaria. Then, we can work backward to the first and simplest “pilot” trials to determine how they can best be designed and conducted to give the maximal useful information to inform the design of those larger, later trials.
For example, a crucial aspect of an initial pilot trial of gene drive will be to determine the rate of spread of the gene drive transgene from release locations. Once this information is established in the field, it can then be used to determine what distances there might need to be between those initial gene drive release locations in pilot trials and release locations in later, more complex trials to evaluate the impact on malaria.
The new paper provides several potential solutions to the design complexities of initial gene drive field trials, including pilot trials that start off on a small scale but can subsequently be integrated sequentially over time into larger cRCTs. Together, the considerations outlined in the article have helped us start developing protocols for field trial releases.
Our aim is to turn those protocols into tangible field trials of gene drive for malaria vector control in Africa within the next five years.
