How to advance environmental risk assessment of gene drive for malaria when it’s never been done before

Published in Malaria Journal last month, “Recommendations for environmental risk assessment of gene drive applications for malaria vector control” is a meeting report that I co-authored alongside nine other Target Malaria experts and 11 external experts from multiple disciplines.
The report’s nine recommendations are the result of six online workshops involving 50 experts from four continents who were invited to consider how best to conduct the next stages of environmental risk assessments (ERA) of gene drive applications. This builds on the on-going work carried out by our Risk and Regulatory teams to identify and quantify risk for the technologies we are developing. An ERA is a process to identify significant risks to the environment and health, estimating their magnitude and likelihood, and defining any risk management that may be required. It’s important to note environmental risk assessments have never been done for gene drive.

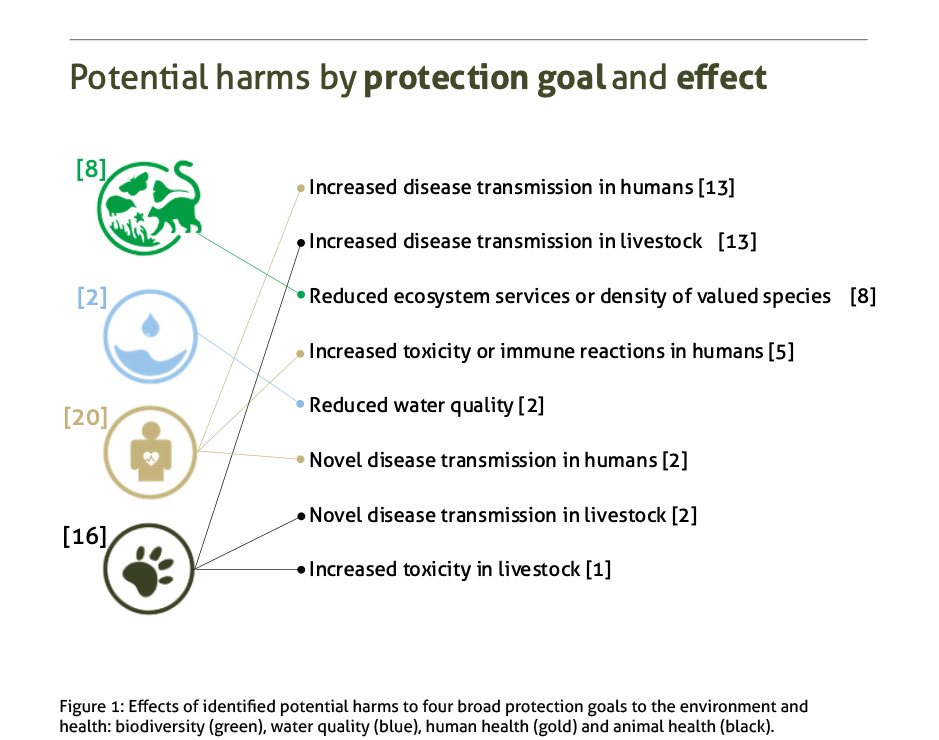
As a first stage of an ERA, we used a ‘problem formulation’ where we defined potential harms and hazards in relation to simulated investigational releases of a doublesex-based gene drive in West Africa. This allowed us to develop 46 ‘pathways’ describing the cause-effect chain of events that could lead to potential harms from a release. Engagement was a constant theme running through all workshops and I will briefly describe the nine recommendations, five of which relate to that theme. The recommendations are discussed in more detail in the report.
Recommendations related to engagement:
- The importance of inclusion of a broad range of expertise to develop guidance that specifies who to engage, when to engage them and why, in ERA for gene drive. (Recommendation One)
- The need for a broad range of expertise in engagement during problem formulation. (Recommendation Four)
- The need for stakeholder input on the development of scenario plans and model representations in ERA for gene drive applications. (Recommendations Seven and Eight)
- Embedding inclusive and effective engagement in the broader framework of gene drive governance. (Recommendation Nine)
Recommendations advancing the technical conduct of ERA for gene drive application:
- How the definition of the term ‘Target Organism’ in the context of a complex of species, such as An. gambiae s.l., needs more nuanced consideration than for other GMO applications. (Recommendation two)
- The importance of using problem formulation that is founded on the protection of specific measurable goals to health and the environment. (Recommendation three)
- The use of the concept of ‘realistic worst case scenarios’ as an entry point into ERA. (Recommendation five)
- The importance of using a range of comparators, from the levels of genotype to intervention type, so that risk can be contextualised. (Recommendation six)
- The desirability of adopting both probabilistic and qualitative approaches to ERA for gene drive applications. (Recommendation seven)
- The importance of developing interaction networks for assessment of ecological risks that have been informed by both biological considerations and stakeholder values. (Recommendation eight)
While recommendation one is aimed at those responsible for producing guidance on ERA, such as decision-makers like national regulators or policymakers in governments or international agencies, the eight other recommendations in the paper are relevant to developers, decision-makers, and policymakers alike.
I hope that these recommendations can contribute to the overall development and successful evaluation of gene drive, and innovative solutions in general, for malaria vector control.
