Summary
- A team of scientists published in Malaria Journal its perspective on how field trials for gene drive mosquitoes to control malaria might be designed and conducted in the next few years, entitled: “Considerations for first field trials of low-threshold gene drive for malaria vector control”.
- The paper analyses the scope, objectives, trial design elements, and approaches to monitoring for initial field releases of gene drive systems, informed by the successful implementation of field trials of biological control agents, as well as other vector control tools.
- The co-authors are from the leading three developers of self-sustaining, or “low-threshold,” gene drive for malaria vector control: Target Malaria, Transmission Zero, and University of California Malaria Initiative (UCMI),
- The work described in the paper was supported by the Foundation for the National Institutes of Health (FNIH).
London, June 7, 2024: Malaria Journal has just published an article entitled “Considerations for first field trials of low-threshold gene drive for malaria vector control”. This publication is the product of a two-year collaboration on the design of field trials for gene drive between several research teams developing gene drive mosquitoes to control malaria in Africa: Target Malaria (Burkina Faso, Ghana, Uganda, Italy, USA, UK), Transmission Zero (Tanzania, UK), Foundation for the National Institutes of Health – FNIH (USA), and University of California Malaria Initiative – UCMI (USA & São Tomé and Príncipe).
Gene drive has the potential to offer a new way of eliminating malaria by targeting the mosquitoes that transmit the disease, as highlighted in WHO’s Global Malaria Programme in the updated technical strategy launched in April 2024. Unlike current interventions such as bed nets or indoor insecticide spraying, gene drive mosquitoes are expected to spread and persist in the Anopheles mosquito populations, with the objective of helping reduce malaria transmission rates.
Dr. John B. Connolly, Senior Regulatory Science Officer for Target Malaria and co-author of the article said: “We hope that the concepts outlined in this paper will contribute to developing a solid framework for testing gene drive in field trials and provide researchers, regulatory stakeholders and public health experts with clear ideas on how to assess the effectiveness of gene drive as a potential tool to fight malaria”.
The paper provides several solutions to the potential complexities surrounding the design of initial gene drive field trials. A key aspect of the work involves identifying how low threshold gene drive is expected to work via a “causal pathway”, beginning with release of the gene drive mosquitoes. that would then lead to increases in the proportion of mosquitoes carrying the gene drive transgene, so that the gene drive would spread through target mosquito populations, leading to reductions in the abundance of target mosquitoes, and which would ultimately lead to decreases in malaria transmission.
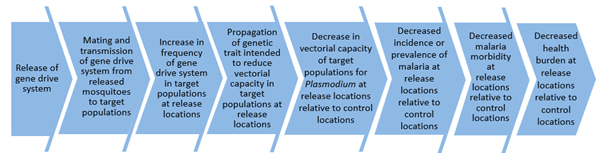
The paper describes how the first field studies of gene drive could be “pilot trials” that are relatively simple and focus on testing earlier points in the causal pathway, or they could be complex and test the earlier and later stages of the causal pathway, including impacts on malaria transmission.
“A fundamental question for decision-makers for the first field trials will be whether there should be a selective focus on earlier points of the pathway, such as genetic efficacy via measurement of the increase in frequency and spread of the gene drive system in target populations, or on wider interrogation of the entire pathway including entomological and epidemiological efficacy”, adds Connolly.
Insights in the paper also come from experiences in field testing of other more convention malaria control interventions. For example, the first trials of a new insecticide-treated bed net might establish whether they reduce mosquito biting rates in a small number of experimental huts. If these are successful, the next step might be to evaluate the direct impact on malaria transmission in much larger, more expensive field trials often involving large numbers of human volunteers.
Likewise, the pilot trial for gene drive might occur on a small scale that might involve only one or two release locations and have only simple goals, perhaps testing early stages in the causal pathway, like how fast the gene drive spreads from release locations. This information on spread could then be used to inform the design of subsequent, larger, more expensive trials measuring later stages of the causal pathway that would have sufficient statistical power to evaluate the impacts of gene drive on malaria transmission.
The paper also describes more sophisticated approaches to initial field trials of low threshold gene drive. Pilot trials designed to obtain data of spread of the gene drive could start off on a small scale but subsequently become integrated sequentially over time into much larger cRCTs measuring impact on malaria. For example, “pilot trials” that start off on a small scale but can subsequently be integrated sequentially over time into larger cluster Randomized Controlled Trials (cRCTs).
