Microinjection: Transformation from wild to genetically modified mosquito


At Target Malaria, our work involves genetically modifying mosquitoes. This gives us valuable insights into the biology of mosquitoes and malaria transmission mechanisms, as well as a way of creating potential species-specific tools that could be used as a tool to help malaria elimination.
Genetic material or “constructs”, designed and created in the lab to produce these modifications, need to be physically delivered into the mosquito, where we can then analyse the effects.
We are often asked how we modify the mosquitoes. In this blog, I outline this process – microinjection.

Photo of the Anopheles gambiae mosquito, the main vector of malaria
Once the injection mixture is prepared, a liquid solution containing copies of the genetic material, it needs to be kept fresh on ice. Then, mosquito eggs are required for the next step.
To produce eggs, the females require nutrients from a blood meal, which they are provided with via an artificial blood-feeding system a few days prior. Since mosquitoes lay their eggs on water in the night, these conditions are mimicked by placing their cage in the dark with a dish of wet paper. Within 15 minutes there are usually enough eggs laid to begin.
Though these are usually called “eggs”, they are more precisely now “embryos” as the females will have already mated with a male and the eggs have been fertilised, with each embryo already starting to develop into a little larva inside its shell.
Once laid, there is a limited window of opportunity for injection – less than an hour! If you are too early, they are too soft and delicate, and if you are too late, the embryos become firm and pressurised inside. The colour of the embryos is a good indicator – freshly laid they are pale yellow-white, and darken over time as they develop pigment, so the in-between colour of light grey is ideal.
For the embryos to stay hydrated and in place during injection, a stereomicroscope (a two-eyepiece scope that allows for a more 3D image compared to a conventional one-eye piece microscope), and brush is used to carefully line them up on a glass microscope slide against a thin nitrocellulose paper, with a wet thicker filter paper on top. The positioning of the embryo and the specific section to be injected are crucial factors to ensure easy access for the needle.
The embryos of mosquitoes and other insects begin with many copies of their genetic material as “nuclei” inside a single cell, that are later compartmentalised into individual cells. Any nuclei/cell could be edited in theory, but it is only those that go on to form the reproductive “germline” cells – i.e if the embryo is female – their future eggs, or if it is male – their future sperm – will the modifications exist in their offspring, passed on to the next generation, and the next… These nuclei are located in the posterior (rear) end of the embryo which can be identified by the slightly thinner and pointier appearance than the opposite anterior (front) end where the head forms. So the embryo is placed with the posterior end at the top.
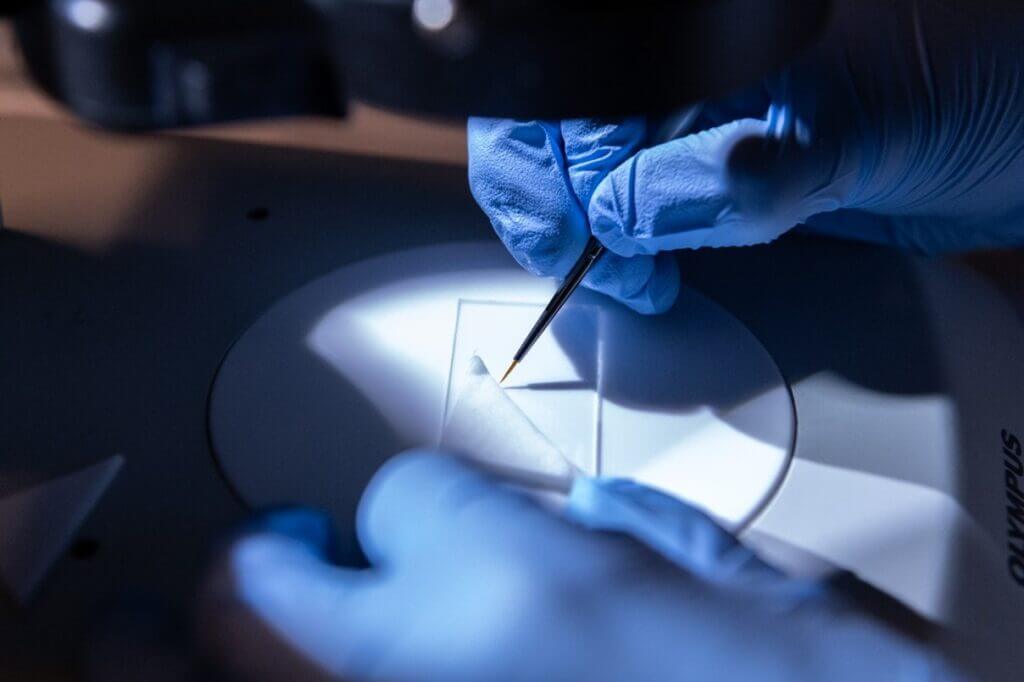
Embryos are transferred into position against a fine paper using a brush
Once lined up in position (~30-50 per slide), the embryos are placed under an inverted microscope (where the light comes from the top and lenses at the bottom, opposite of a conventional microscope) which allows easy visualisation.
As the eggs are so small (about half a millimetre), injections would be impossible to do by hand, so specialised attached equipment is used to control the precise movements of a needle and flow of the injection mixture.
A very fine needle made of quartz glass, also made in the lab, is loaded with the injection mixture, and secured into place in a holder, with a supply of air pressure provided through tubing and a pump from the back to control liquid flow. Needle movements are controlled via a joystick, and injection pressure is controlled via a computer control box and mouse. With a click of a button, the mixture is injected, which can be noticed as a small movement of the contents inside the embryo.


Loading the microinjection needle with the mix
Microinjecting the embryos
Much care is needed for the embryos to receive enough solution to introduce the genetic material in the right place without causing damage. This is a specific skill that can take a while to learn.

Embryos being injected
After being injected, the embryos are placed into a pot of water surrounded by wet paper. Over the next two days, they develop into larvae and start hatching, wriggling away in the water.
Since the modifications are not visible, we also include a gene that produces a fluorescent protein that glows under a microscope with a laser light at a specific wavelength. This fluorescence acts as a “marker” and allows tracking of the genetic modifications.
When the injected embryos hatch as larvae, they may show “transient” expression of this marker at the site of injection, indicating injection success.
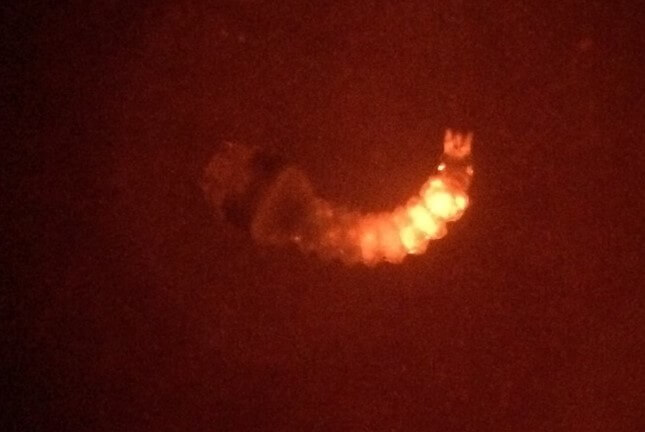
Injected mosquito hatched as a larva showing transient expression
As mentioned, the genetic material must not only be present but integrated into their germline cells to be passed on to the next generation. So once adult, the injected mosquitoes are all outcrossed to those of the opposite sex (normally wild-types to maximise numbers) and their progeny/offspring are screened for those that are “transgenic” – ie that have fully inherited the modifications.
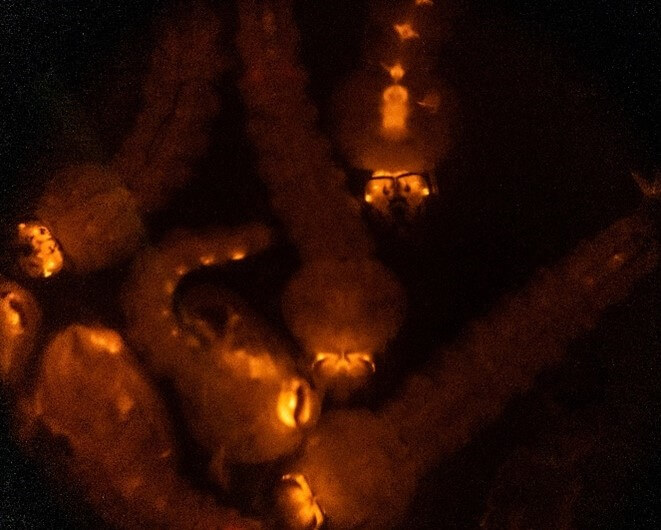
Offspring of injected mosquitoes showing transgenic expression
Transgenics are checked molecularly in the lab to ensure the genetic material has integrated as intended. Then studying the effects of the modifications can now commence…
