Investigating potential harms from the use of gene drive mosquitoes to fight malaria

Target Malaria’s mission is to develop and share new, cost-effective and sustainable genetic technologies to modify mosquitoes and reduce malaria transmission in Africa. With the technology still in progress in the labs, the project’s approach is deliberately cautious and iterative. Central to this is Environmental Risk Assessment (ERA) that evaluates the potential impacts that the release of gene drive mosquitoes could have on the environment or on human and animal health. This ongoing area of work is carried out by our Risk and Regulatory teams and builds upon numerous other pieces of work and publications in this area, and builds on the recommendations of the World Health Organization:
“Candidate genetically modified mosquitoes considered for progression to any level of field testing should be assessed thoroughly for all possible hazards and demonstrate efficacy and fitness in the laboratory that are consistent with the desired disease reduction goal”

We have recently published a paper in Malaria Journal entitled “Systematic identification of plausible pathways to potential harm via problem formulation for investigational releases of a population suppression gene drive to control the human malaria vector Anopheles gambiae in West Africa”. It is based on the simulated release of the gene drive mosquito strain carrying the doublesex transgene (dsxFCRISPRh) developed at the lab at Imperial College London, which has already been shown to reduce caged mosquito populations, as described in Kyrou et al., 2018.
Our paper used “problem formulation”, a rigorous scientific analysis that is an initial step in Environmental Risk Assessments, to identify potential harms to “protection goals”. These are objectives for the environment or animal and human health that are considered of particular value to the regions where the releases would occur. The paper considers potential harms to four protection goals: human health, animal health, biodiversity and water quality.
Our analysis drew upon publications from a series of four regional workshops held in Africa, as well as another in the USA, between 2016 and 2019; organized by the New Partnership for Africa’s Development of the African Union Development Agency (AUDA-NEPAD) and by the Foundation for the National Institutes of Health (FNIH). It was further complemented by extensive literature assessment, and ongoing dialogue with numerous scientific, risk and regulatory experts.
We systematically identified 46 potential harms to those protection goals and defined the plausible pathways, or causal chain of events, by which they could occur. We then developed risk hypotheses to test the validity of key individual steps in each of these pathways and outlined further studies that should in future allow us to either accept or reject each of those risk hypotheses.
The process supports the development of a formal risk assessment to submit to national regulatory authorities. It plays an important part in ensuring that the final design of a gene drive vector control system is safe.
The exercise revealed that:
- In many cases, the more the mosquito population is reduced by gene drive mosquitoes, the less likely potential harms are to be realised.
- The most common potential harms that were identified involved increased human or animal disease transmission.
- The developed pathways will inform the next stages of an ERA on population suppression gene drive, which will involve evaluation of the likelihood and magnitude of each of the identified potential harms.
- Future regulatory science studies that can dissect and evaluate the capacity of a population of mosquitoes to transmit malaria – such as comparison of biting rates and vector competence in genetically modified mosquitoes with wild mosquitoes – will be crucial for subsequent stages of the ERA.
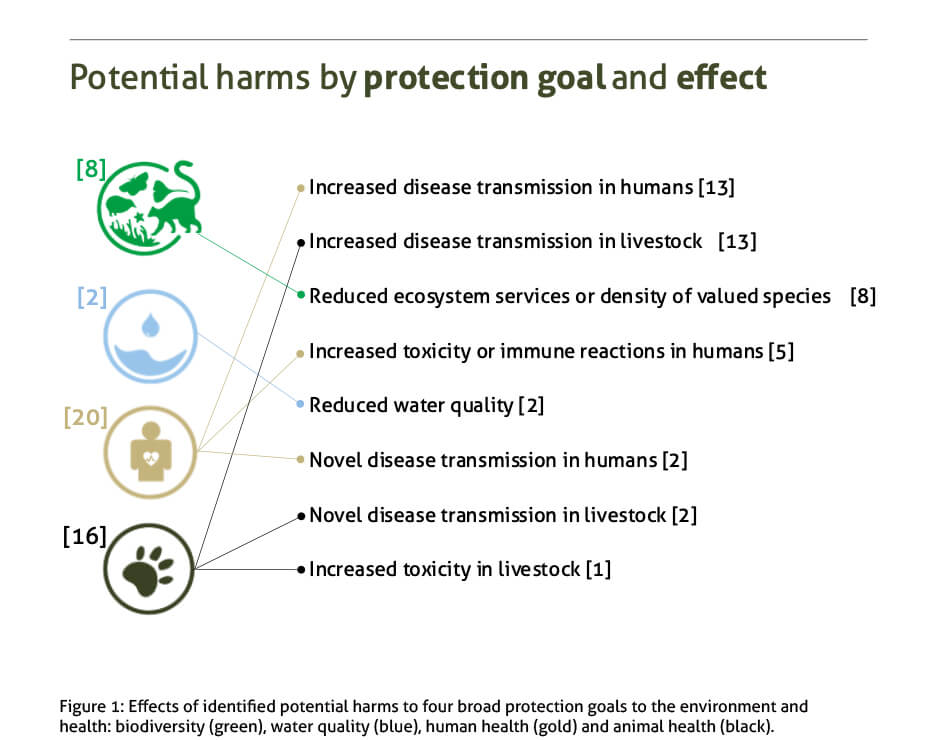
To our knowledge, our paper represents, the first systemic and comprehensive identification of the potential harms that gene drive mosquitoes could have on the environment and on human health, should they be released.
We hope that it will also stimulate further, broader engagement on the use of population suppression gene drive to control malaria vectors in Africa. With half a million deaths caused by malaria every year, new tools are sorely needed.
