New study on cluster randomised controlled trials for mosquito release interventions such as gene drive


New technologies for suppressing populations of disease-transmitting mosquitoes involve releasing modified male mosquitoes into field environments.
One example of these technologies is the sterile insect technique (SIT) which releases male mosquitoes that have been sterilised using radiation treatment. A second one is genetically modified mosquitoes, including gene drive mosquitoes, which are a potential candidate tool for malaria control.
Interest in mosquito release interventions is rapidly growing, spurred by recent global rises in cases of mosquito-borne diseases, and stalling progress in malaria control. Recent field trials have shown these technologies to be very effective in reducing dengue incidence, while very few field trials have yet been conducted to test how effective they could be in malaria reduction.
In a new study titled “Requirements for designing cluster randomised control trials to detect suppression of malaria vector”, our research teams in Burkina Faso and the United Kingdom have investigated how to evaluate the efficacy of an intervention in suppressing malaria vector populations using cluster randomised controlled trials (CRCTs).
Cluster randomised controlled trials (CRCTs) are the gold standard for assessing intervention efficacy, comparing intervention performance in a series of clusters or communities that receive it against a series of clusters that don’t receive the intervention and serve as controls. If the efficacy of mosquito release interventions could be demonstrated by a CRCT, this would be a major step forward for these new technologies for vector control.
In our study, we used a rich data set of mosquito population densities collected from field sites in western Burkina Faso to develop statistical models to inform the design of CRCTs for suppressing malaria-transmitting mosquito populations. By considering the natural variability in mosquito densities over time, and between different locations, we investigated how to efficiently design trials so that the suppressing effect of the intervention could be detected with minimal sampling effort. We found that as few as 20-21 clusters were required to detect a suppression effect of 50%, while 9-10 clusters were required to detect a larger suppression effect of 90%.
This research is timely in contributing to the design of field trials of gene drive releases, which are currently being actively planned. CRCT designs do not need a large number of clusters to detect moderate to high suppression effects acting on An. gambiae malaria vector populations.
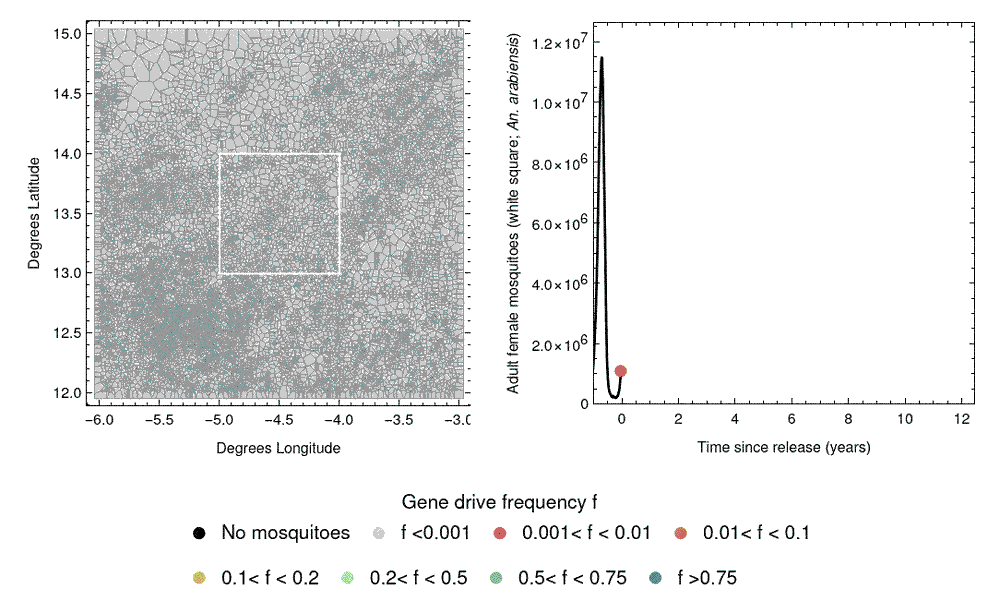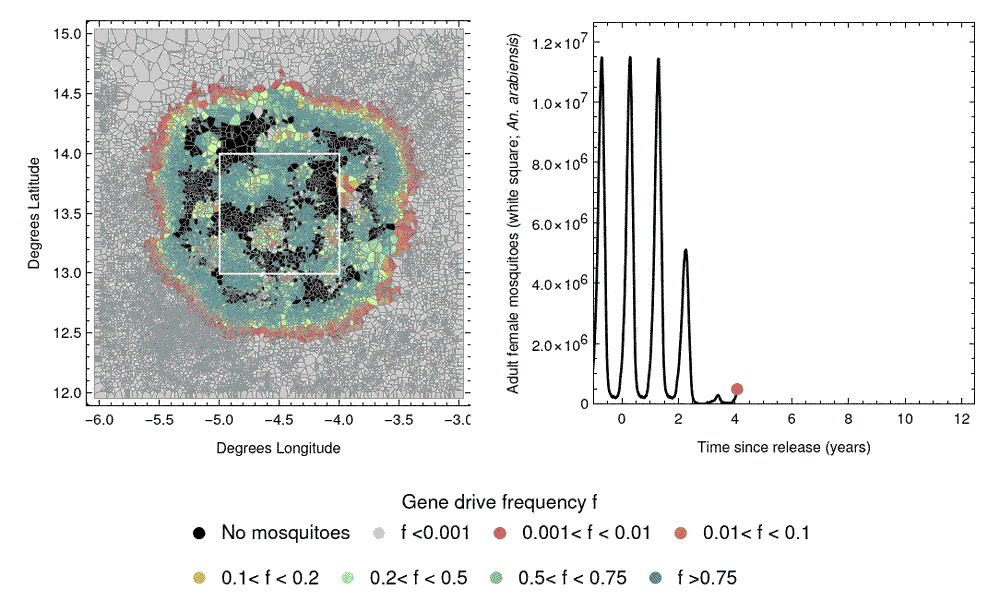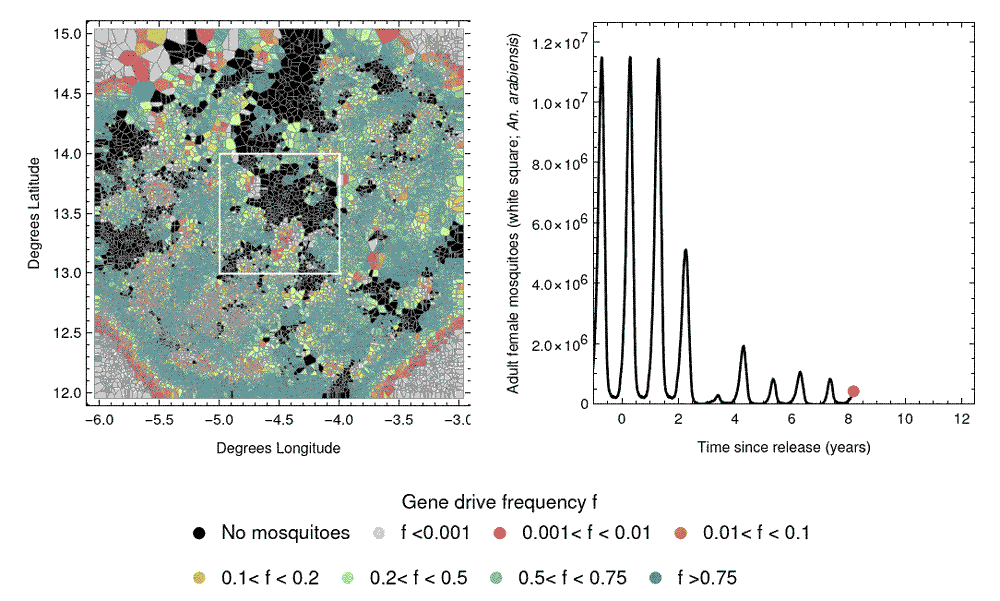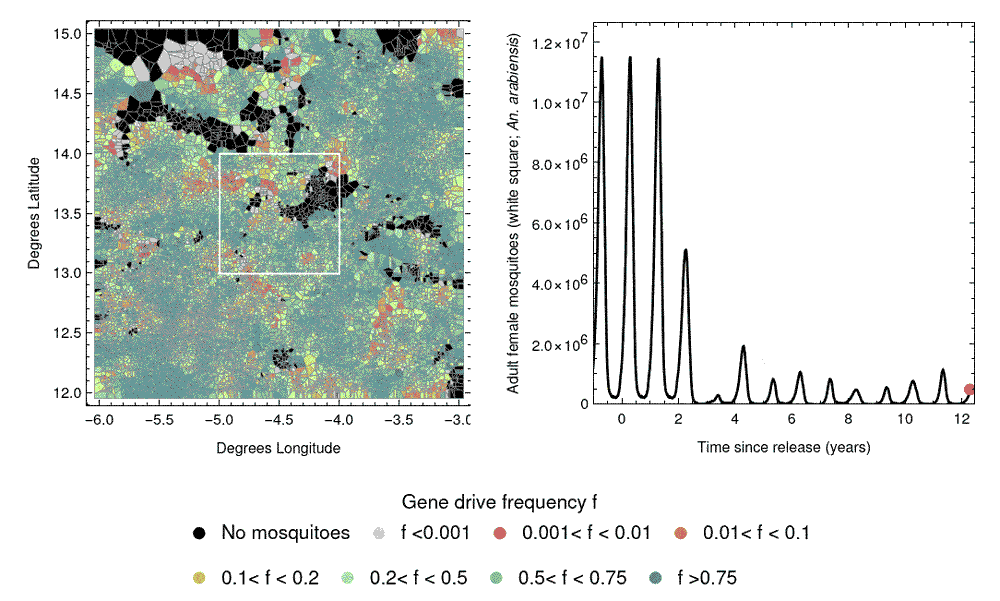
A challenge in designing field trials of gene drive releases involves the spatial spread of gene drives, due to mosquito dispersal. This could potentially carry the gene drive from release sites into control clusters, causing “spillover”, which could compromise the study design. The next phase of our work will involve developing mathematical models to predict the spatial spread of gene drives, and how this might affect the performance of CRCTs. This can further inform CRCT design and help us select a spatial arrangement of clusters to mitigate the influence of spillover on the trial results.